A) IV > III > II > I
B) IV > I > III > II
C) I > II > III > IV
D) IV > II > III > I
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is pure acetic acid often called glacial acetic acid?
A) Because it freezes just below 0°C,forming white crystals
B) Because it freezes just below 100°C,forming white crystals
C) Because it freezes just below room temperature,forming white crystals
D) Because it freezes just above room temperature,forming white crystals
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the following compounds in order of increasing boiling point,putting the compound with the lowest boiling point first. 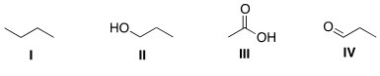
A) I < II < III < IV
B) I < IV < II < III
C) III < II < IV < I
D) II < IV < I < III
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the carbon atom in a carboxy group?
A) sp
B) sp2
C) sp3
D) p
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the IUPAC name for the following compound. 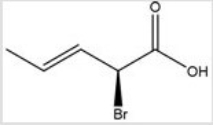
A) (E,R) -2-bromo-3-pentenoic acid
B) (Z,R) -2-bromo-3-pentenoic acid
C) (E,S) -2-bromo-3-pentenoic acid
D) (Z,S) -2-bromo-3-pentenoic acid
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is least basic? 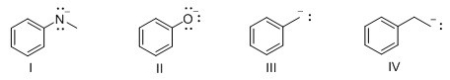
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the overall charge of the amino acid,alanine,at pH = 7?
A) + 1
B) - 1
C) No overall charge
D) + 2
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What two strong absorptions are characteristic of the IR spectrum of carboxylic acids?
A) A C=O absorption at 1710 cm-1 and a C-H absorption at 3000 cm-1.
B) A C=O absorption at 1710 cm-1 and an O-H absorption at 2500-3500 cm-1.
C) A C=O absorption at 1600 cm-1 and an O-H absorption at 2500-3000 cm-1.
D) A C-O absorption at 1500 cm-1 and an O-H absorption at 2500-3500 cm-1.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What physical property and reaction type are used by extraction as useful techniques to separate and purify mixtures of compounds?
A) Physical property = solubility differences; reaction type = acid-base reaction
B) Physical property = boiling point; reaction type = acid-base reaction
C) Physical property = solubility differences; reaction type = oxidation-reduction
D) Physical property = density; reaction type = oxidation-reduction
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of increasing acidity,putting the least acidic first. 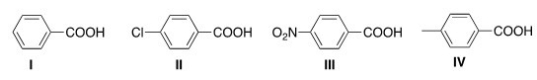
A) I < II < III < IV
B) III < II < I < IV
C) IV < I < II < III
D) I < IV < III < II
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is most basic? 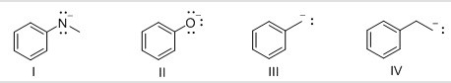
A) I
B) II
C) III
D) IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would happen if a mixture of benzoic acid (C6H5COOH) and NaCl is added to a separatory funnel containing H2O and CH2Cl2?
A) The benzoic acid would dissolve in the water layer and the NaCl would dissolve in the organic layer.
B) The benzoic acid would dissolve in the organic layer and the NaCl would dissolve in the water layer.
C) Both benzoic acid and NaCl would dissolve in the organic layer.
D) Both benzoic acid and NaCl would dissolve in the water layer.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the overall charge of the amino acid,alanine,at pH = 10?
A) + 1
B) - 1
C) No overall charge
D) - 2
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 53 of 53
Related Exams