A) I
B) II
C) III
D) IV
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkyl halides undergoes the fastest SN2 reaction with sodium hydroxide?
A) 1-Iodobutane
B) 1-Chlorobutane
C) 1-Fluorobutane
D) 1-Bromobutane
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the alkyl halides in order of decreasing SN2 reactivity,putting the most reactive first. 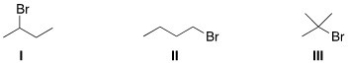
A) I > II > III
B) II > I > III
C) III > I > II
D) I > III > II
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements explain why aryl halides and vinyl halides do not undergo nucleophilic substitution by either the SN1 or SN2 mechanism?
A) They don't undergo SN1 reactions because a higher percent s-character makes the bond longer and stronger.
B) They don't undergo SN2 reactions because a higher percent s-character makes the bond shorter and stronger.
C) They don't undergo SN2 reactions because heterolysis of the C-X bond forms a highly unstable carbocation.
D) They don't undergo SN1 reactions because the carbocation is highly electronegative.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the Hammond postulate is true?
A) In an exothermic reaction,lowering the energy of the transition state increases the activation energy,Ea.
B) In an endothermic reaction,the more stable product forms faster.
C) In an endothermic reaction,the less stable product forms faster.
D) In an endothermic reaction,the activation energy,Ea,is similar for both products.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 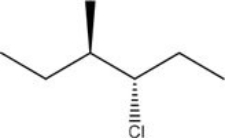
A) (3R,4R) -3-chloro-4-methylhexane
B) (3R,4S) -3-chloro-4-methylhexane
C) (3S,4R) -3-chloro-4-methylhexane
D) (3S,4S) -3-chloro-4-methylhexane
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the nucleophilic substitution reaction shown below? 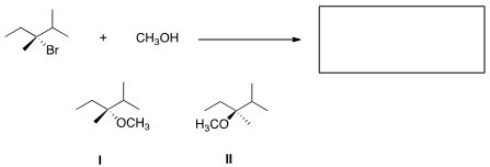
A) Only I
B) Only II
C) I and II
D) None
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following in order of decreasing nucleophilicity,putting the most nucleophilic first. 
A) II > III > I > IV
B) III > II > IV > I
C) II > III > IV > I
D) III > II > I > IV
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the nucleophilic substitution reaction shown below? 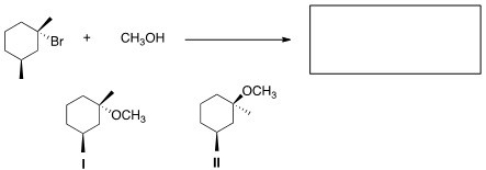
A) Only I
B) Only II
C) I and II
D) None
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the nucleophilic substitution reaction shown below? 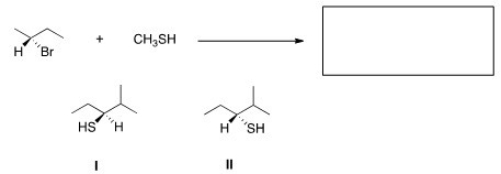
A) Only I
B) Only II
C) I and II
D) None
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about SN2 reactions is true?
A) The rate of reaction is dependent on just the substrate.
B) The fastest reaction will occur with a tertiary alkyl halide.
C) The mechanism is a two-step process.
D) Displacement occurs with inversion of configuration.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkyl halides is a tertiary alkyl halide? 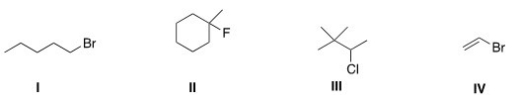
A) I
B) II
C) III
D) IV
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of increasing SN1 reactivity? 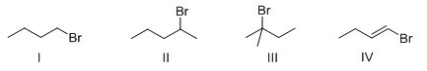
A) I > II > III > IV
B) IV > I > II > III
C) IV > III > II > I
D) III > II > I > IV
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkyl halides is a primary alkyl halide? 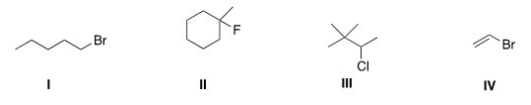
A) I
B) II
C) III
D) IV
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 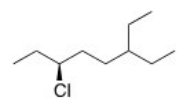
A) (R) -3-Chloro-6-ethyloctane
B) (S) -3-Chloro-6-ethyloctane
C) (S) -6-Chloro-3-ethyloctane
D) (R) -6-Chloro-3-ethyloctane
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures have the correct common name? 
A) I and II
B) II and III
C) II and IV
D) III and IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following ions in order of increasing nucleophilicity in polar protic solvents,starting with the least nucleophilic ion. 
A) I < II < III < IV
B) IV < III < II < I
C) I < II < IV < III
D) IV < III < I < II
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following substitution reaction,what would be the effect of changing the solvent from CH3OH to (CH3) 2S=O? CH3(CH2) 5Br + NaOH ® CH3(CH2) 5OH + Br-
A) The rate would decrease because SN1 reactions are favored by polar protic solvents.
B) The rate would increase because SN2 reactions are favored by polar aprotic solvents.
C) The rate would increase because SN1 reactions are favored by polar protic solvents.
D) The rate would decrease because SN2 reactions are favored by polar aprotic solvents.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the nucleophilic substitution reaction shown below? 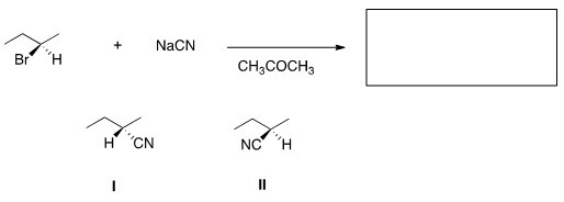
A) Only I
B) Only II
C) I and II
D) None
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true?
A) Sodium ethoxide is a better nucleophile than sodium tert-butoxide.
B) Sodium tert-butoxide and sodium ethoxide have similar strengths as bases.
C) Sterically hindered bases are also called nonnucleophilic bases.
D) Steric hindrance decreases basicity but not nucleophilicity.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 73
Related Exams