A) N--C
B) N--Si
C) N--P
D) N--Al
E) N--Ga
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules or ions will have a Lewis structure most like that of phosphorus trichloride,PCl3?
A) ClO3-
B) SO3
C) CO32-
D) BF3
E) Cl2CO
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the Lewis electron dot structure for carbon monoxide?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formal charge of the oxygen atom in the Lewis structure for cyanate shown below? 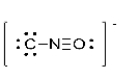
A) 0
B) −2
C) +1
D) −1
E) 2
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules or ions are isoelectronic: SO3,NF3,NO3-,CO32-?
A) SO3 and NF3
B) NF3 and CO32-
C) SO3,NF3 and CO32-
D) SO3,NO3- and CO32-
E) SO3,NF3,NO3-,and CO32-
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct Lewis dash formula for carbon diselenide?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the best Lewis structure of CH2FCO2H (connectivity correct as given) ?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) none of these is acceptable
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose which central atom in the following molecules is most electronegative.
A) PH3
B) CH4
C) H2S
D) H2O
E) NH3
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following groups of molecules contains no ionic compounds?
A) HCN,NO2,and Ca(NO3) 2
B) PCl5,LiBr,and Zn(OH) 2
C) KOH,CCl4,and SF4
D) NaH,CaF2,and NaNH2
E) CH2O,H2S,and NH3
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules is nonpolar?
A) sulfur dioxide,SO2
B) hydrogen fluoride,HF
C) phosphorus trifluoride,PF3
D) boron trifluoride,BF3
E) iodine trichloride,ICl3
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In molecules,as bond order increases,
A) both bond length and bond energy increase.
B) both bond length and bond energy decrease.
C) bond length increases and bond energy is unchanged.
D) bond length is unchanged and bond energy increases.
E) bond length decreases and bond energy increases.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory,which of the following is the correct molecular geometry around the carbon atom in formaldehyde?
A) Linear
B) Bent
C) Trigonal-planar
D) Tetrahedral
E) Octahedral
G) A) and B)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Based on electron-pair geometries,which of the following molecules has the smallest bond angle between any two adjacent atoms?
A) CH4
B) H2O
C) BH3
D) PH3
E) SF6
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When both of the electrons in a molecular bond originate from the same atom,the bond is called a(n)
A) sigma bond.
B) coordinate covalent bond.
C) pi bond.
D) metallic bond.
E) ionic bond.
G) A) and C)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
The central atom in SF6 is surrounded by
A) six single bonds and no lone pairs of electrons.
B) six single bonds and one lone pair of electrons.
C) six single bonds and two lone pairs of electrons.
D) five single bonds,one double bond,and one lone pair of electrons.
E) four single bonds,two double bonds,and no lone pairs of electrons.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is able to form a molecular structure that exceeds the octet rule?
A) C
B) N
C) O
D) F
E) Cl
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the approximate H−N−H bond angles in NH4+?
A) 109.5°
B) 120°
C) 109.5° and 120°
D) 90°
E) 90° and 120°
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct Lewis structure for Cl2CO is (the 2 Cl's and the O are bound to the C and not to each other) :
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) none of these
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of nitrogen trichloride,NCl3.
A) The electron-pair geometry is linear,the molecular geometry is linear.
B) The electron-pair geometry is trigonal-planar,the molecular geometry is trigonal-planar.
C) The electron-pair geometry is trigonal-planar,the molecular geometry is bent.
D) The electron-pair geometry is tetrahedral,the molecular geometry is tetrahedral.
E) The electron-pair geometry is tetrahedral,the molecular geometry is trigonal-pyramidal.
G) A) and E)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
What is the bond angle in a trigonal planar molecule or ion?
A) 109°
B) 180°
C) 90°
D) 72°
E) 120°
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 89
Related Exams