A) 5
B) 4
C) 1
D) 2
E) 3
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide:
 In a particular experiment, a 5.00- g sample of CaO is reacted with excess water and 6.11 g of Ca(OH) 2 is recovered. What is the percent yield in this experiment?
In a particular experiment, a 5.00- g sample of CaO is reacted with excess water and 6.11 g of Ca(OH) 2 is recovered. What is the percent yield in this experiment?
A) 7.19
B) 122
C) 81.9
D) 1.22
E) 92.4
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula weight of calcium nitrate (Ca(NO3) 2) is _ amu.
A) 150.1
B) 204.2
C) 102.1
D) 116.1
E) 164.0
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the following equation is balanced, the coefficient of dinitrogen pentoxide is . 
A) 2
B) 1
C) 5
D) 3
E) 4
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the following equation is balanced, the coefficient of Al is . 
A) 3
B) 1
C) 5
D) 4
E) 2
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction used to inflate automobile airbags _.
A) is a decomposition reaction
B) produces sodium gas
C) violates the law of conservation of mass
D) is a combination reaction
E) is a combustion reaction
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following substances is the product of this combination reaction? Al (s) + I2 (s) -
A) AlI
B) AlI3
C) Al2I3
D) Al3I2
E) AlI2
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The combustion of ammonia in the presence of excess oxygen yields NO2 and H2O:
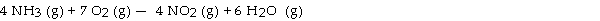 The combustion of 43.9 g of ammonia produces g of NO2.
The combustion of 43.9 g of ammonia produces g of NO2.
A) 2.58
B) 178
C) 43.9
D) 0.954
E) 119
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the reactions below, which one is not a combination reaction?
A) 2CH4 + 4O2 -2CO2 + 4H2O
B) CaO + H2O - Ca(OH) 2
C) 2Mg + O2 - 2MgO
D) C + O2 - CO2
E) 2N2 + 3H2 - 2NH3
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the following equation is balanced, the coefficient of H2 is _ . 
A) 4
B) 3
C) 2
D) 0
E) 1
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The combustion of propane (C3H8) in the presence of excess oxygen yields CO2 and H2O:
 When 2.5 mol of O2 are consumed in their reaction, mol of CO2 are produced.
When 2.5 mol of O2 are consumed in their reaction, mol of CO2 are produced.
A) 2.5
B) 5.0
C) 3.0
D) 1.5
E) 6.0
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of C3H8O that contains 200 molecules contains _ carbon atoms.
A) 4.01 × 1025
B) 1.20 × 1026
C) 600
D) 200
E) 3.61 × 1026
G) B) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The quantity of product that is calculated to form when all of the limiting reagent reacts is called the actual yield.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the following equation is balanced, the coefficient of H2O is _ . 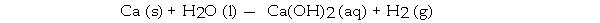
A) 4
B) 1
C) 5
D) 3
E) 2
G) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
Complete and balance the following reaction, given that elemental rubidium reacts with elemental sulfur to form Rb2S (s). Na (s) + S (s) -
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula weight of silver chromate (Ag2CrO4) is _ amu.
A) 159.87
B) 175.87
C) 223.87
D) 331.73
E) 339.86
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Under appropriate conditions, nitrogen and hydrogen undergo a combination reaction to yield ammonia:
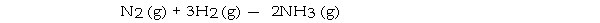 A 7.1- g sample of N2 requires g of H2 for complete reaction.
A 7.1- g sample of N2 requires g of H2 for complete reaction.
A) 17.2
B) 0.76
C) 1.2
D) 1.5
E) 0.51
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the following equation is balanced, the coefficient of Al2O3 is _ . 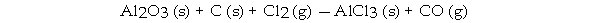
A) 5
B) 3
C) 4
D) 1
E) 2
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Combustion of a 1.031- g sample of a compound containing only carbon, hydrogen, and oxygen produced 2.265 g of CO2 and 1.236 g of H2O. What is the empirical formula of the compound?
A) C3H5O
B) C3H8O
C) C3H9O3
D) C6H16O2
E) C3H6O3
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound is composed of only C, H, and O. The combustion of a 0.519- g sample of the compound yields 1.24 g of CO2 and 0.255 g of H2O. What is the empirical formula of the compound?
A) C6H6O
B) CH3O
C) C3H3O
D) C2H6O2
E) C2H6O5
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 134
Related Exams