Filters
Question type
A) 0.020 M BaCl2
B) 0.020 KCl
C) pure water
D) 0.020 AgNO3
E) 0.015 NaCl
F) A) and B)
G) A) and C)
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Question 52
Multiple Choice
Consider the following table of Ksp values. 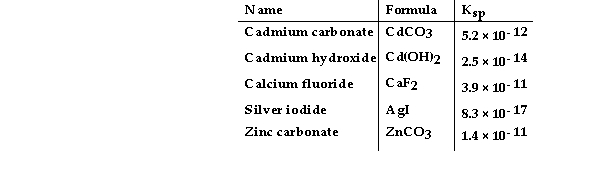 -Which compound listed below has the smallest molar solubility in water?
-Which compound listed below has the smallest molar solubility in water?
A) CdCO3
B) CaF2
C) Cd(OH) 2
D) ZnCO3
E) AgI
F) A) and B)
G) B) and C)
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Question 53
Multiple Choice
How many milliliters of 0.0850 M NaOH are required to titrate 25.0 mL of 0.0720 M HBr to the equivalence point?
A) 3.92
B) 21.2
C) 0.245
D) 0.153
E) 29.5
F) D) and E)
G) A) and D)
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Question 54
Short Answer
200.0 ml of a solution containing 0.5000 moles of acetic acid per liter is added to 200.0 ml of 0.5000 M NaOH. What is the final pH? The Ka of acetic acid is 1.770 × 10- 5.
Correct Answer

verified
Correct Answer
verified
Question 55
Multiple Choice
What is the pH of a buffer solution that is 0.211 M in lactic acid and 0.111 M in sodium lactate? The Ka of lactic acid is 1.37 × 10- 4.
A) 4.13
B) 3.57
C) 5.48
D) 14.28
E) 10.43
F) B) and C)
G) A) and B)
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Question 56
Multiple Choice
What is the molar solubility of magnesium carbonate ( MgCO3 ) in water? The solubility- product constant for MgCO3 is 3.5 × 10- 8 at 25°C.
A) 7.0 × 10- 8
B) 1.8 × 10- 8
C) 1.9 × 10- 4
D) 7.46
E) 2.6 × 10- 4
F) All of the above
G) C) and D)
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Question 57
Short Answer
Suppose you have just added 200.0 ml of a solution containing 0.5000 moles of acetic acid per liter to 100.0 ml of 0.5000 M NaOH. What is the final pH? The Ka of acetic acid is 1.770× 10- 5.
Correct Answer

verified
Correct Answer
verified
Question 58
Multiple Choice
A solution is prepared by dissolving 0.23 mol of hydrazoic acid and 0.27 mol of sodium azide in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of NaOH to this buffer solution causes the pH to increase slightly. The pH does not increase drastically because the NaOH reacts with the present in the buffer solution. The Ka of hydrazoic acid is 1.9 × 10- 5.
A) azide
B) This is a buffer solution:
The pH does not change upon addition of acid or base.
C) H3O+
D) H2O
E) hydrazoic acid
F) C) and E)
G) A) and B)
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 88 of 88
Related Exams