A) 1.67 x 1024 molecules
B) 5.42 x 1026 molecules
C) 2.16 x 1023 molecules
D) 3.01 x 1025 molecules
E) 8.30 x 10-23 molecules
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molar mass of PH3.
A) 40.11 g/mol
B) 31.98 g/mol
C) 33.99 g/mol
D) 93.92 g/mol
E) 2.05 x 1025 g/mol
G) C) and D)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
If the molar mass of a substance is 20.0 g/mol, what is the mass of 4.01 x 1023 molecules of the substance?
A) 30.0 g
B) 13.3 g
C) 8.02 x 1024 g
D) 1.20 x 1025 g
E) 3.00 g
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order from least chlorine atoms to most chlorine atoms in a 50.0 g sample: Cl2, ClF3, Cl2O, PCl3
A) ClF3 < Cl2O < Cl2 < PCl3
B) PCl3 < Cl2O < Cl2 < ClF3
C) ClF3 < PCl3 < Cl2O < Cl2
D) ClF3 < PCl3 < Cl2 < Cl2O
E) Cl2O < Cl2 < ClF3 < PCl3
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the molar mass of a substance is 44.01 g/mol, what is the mass of 1.05 x 1024 molecules of the substance?
A) 76.7 g
B) 1.74 g
C) 4.62 x 1025 g
D) 4.19 x 10-23 g
E) 25.2 g
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the molecules in the figure have an empirical formula that is different from their molecular formula? 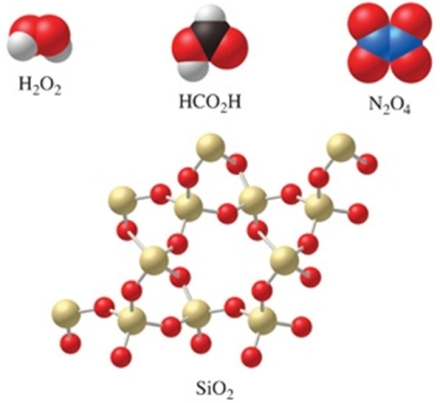
A) H2O2, N2O4, and HCO2H
B) SiO2
C) H2O2 and N2O4
D) all of the compounds
E) none of the compounds
G) A) and B)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Calculate the molar mass of C3H6Cl2.
A) 155.08 g/mol
B) 77.54 g/mol
C) 48.47 g/mol
D) 72.49 g/mol
E) 112.98 g/mol
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much water had to be added to 10.0 mL of the first solution to obtain the second solution? 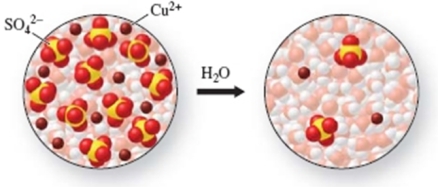
A) 10.0 mL
B) 20.0 mL
C) 40.0 mL
D) 50.0 mL
E) 500.0 mL
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
5.06 moles of iron(II) phosphate, Fe3(PO4) 2, are produced in a reaction.What mass of iron(II) phosphate is produced?
A) 520.g
B) 20.3 g
C) 1.81 x 103 g
D) 3.05 x 1024 g
E) 70.7 g
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 100.0 g sample of a compound contains 1.37 x 1024 molecules.Which of the following could be this compound?
A) CO2
B) NH3
C) CH4
D) CCl4
E) CF4
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many molecules of CO2 are present in 0.100 mol of CO2?
A) 6.02 x 1022 molecules
B) 4.40 molecules
C) 1.66 x 10-25 molecules
D) 6.02 x 1024 molecules
E) 0.100 molecules
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 50.0 mL of 0.235 M NaCl solution is diluted to 200.0 mL, what is the concentration of the diluted solution?
A) 0.0588 M
B) 0.0118 M
C) 58.8 M
D) 1.18 M
E) 0.0426 M
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many formula units are there in 2.5 moles of MgCl2?
A) 2.5
B) 7.5
C) 1.5 x 1024
D) 4.5 x 1024
E) 4.2 x 10-24
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 6.25 g sample of magnetite (Fe3O4) contains 4.52 g of Fe.What are the percentages of iron and oxygen in magnetite?
A) 28.6% Fe and 71.4% O
B) 42.8% Fe and 57.2% O
C) 72.3% Fe and 27.7% O
D) 27.7% Fe and 72.3% O
E) 57.2% Fe and 42.8% O
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many hydrogen atoms are there in 2.0 moles of CH4?
A) 8.0
B) 2.0
C) 1.2 x 1024
D) 1.3 x 10-23
E) 4.8 x 1024
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following in order of increasing mass: 1.0 mole of methane (CH4) , 0.50 mole of water (H2O) , 0.20 mole of Fe, and 0.010 mole of U.
A) U < Fe < H2O < CH4
B) H2O < CH4 < Fe < U
C) H2O < CH4 < U < Fe
D) U < H2O < CH4 < Fe
E) U < H2O < Fe < CH4
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the substances in the figure from least atoms per mole to most atoms per mole. 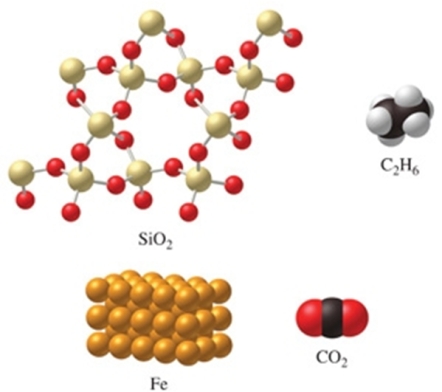
A) SiO2 < C2H6 < CO2 < Fe
B) C2H6 < SiO2 = CO2 < Fe
C) Fe < CO2 = SiO2 < C2H6
D) Fe < CO2 < SiO2 < C2H6
E) C2H6 < SiO2 < CO2 < Fe
G) A) and C)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following statements regarding empirical and molecular formulas is correct?
A) Information on percent composition is all that is necessary to determine both an empirical and a molecular formula.
B) A compound with an empirical formula of CH2O and a molar mass of 90 g/mol would have a molecular formula of C3H6O3.
C) The compounds C2H4 and C3H6 have different empirical formulas.
D) The formula Ca2Cl4 is the correct empirical formula for calcium chloride.
E) The compound benzene has a molecular formula of C6H6, so its empirical formula would be C3H3.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical reaction produces 7.25 moles of barium sulfate, BaSO4.What mass of barium sulfate is produced?
A) 185 g
B) 233 g
C) 1.69 x 103 g
D) 31.1 g
E) 3.11 x 10-2 g
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding atoms, molecules, and moles is correct?
A) Chemists are inherently lazy, so they weigh substances in order to avoid counting out the atoms or molecules in a sample.
B) It would be possible for an individual to count out a mole of atoms or molecules if they had a few days to do it.
C) A single grain of sand has about as many formula units of SiO2 as there are sand grains on all of the beaches on Earth.
D) A mole of HCl would have the same mass as a mole of NaCl, since they have the same number of particles.
E) Since a mole of LiCl has a mass of 42.39 g, the average mass of a LiCl formula unit would be 42.39 mole.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 111
Related Exams