A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The strongest interactions between atoms of helium, He, are examples of
A) ionic bonds.
B) covalent bonds.
C) hydrogen bonds.
D) dipole-dipole attractions.
E) dispersion forces.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The strongest interactions in the compound sodium fluoride, NaF, are examples of
A) ionic bonds.
B) covalent bonds.
C) hydrogen bonds.
D) dipole-dipole attractions.
E) dispersion forces.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many valence electrons are in the Lewis structure of 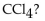
A) 6
B) 0
C) 32
D) 8
E) 82
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrogen sulfide, 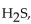 has a shape similar to
has a shape similar to
A) carbon dioxide.
B) carbon tetrachloride.
C) water.
D) carbon monoxide.
E) hydrogen chloride.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substances contains a nonpolar covalent bond?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct formula for the iron(II) ion?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name of 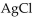 is
is
A) sulfur(I) chloride.
B) silver(I) chloride.
C) gold(I) chloride.
D) silver(II) chloride.
E) silver chloride.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The strongest interactions between molecules of iodine, 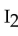 , are examples of
, are examples of
A) ionic bonds.
B) covalent bonds.
C) hydrogen bonds.
D) dipole-dipole attractions.
E) dispersion forces.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A(n) ________ is the smallest unit of two or more atoms held together by a covalent bond.
A) ionic compound
B) unit
C) nucleus
D) molecule
E) formula
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct formula for iron(III) sulfide?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following polyatomic ions has a positive charge?
A) hydroxide
B) sulfate
C) hydrogen carbonate
D) nitrate
E) ammonium
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula of carbon tetraiodide?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements has the lowest electronegativity?
A) N
B) F
C) O
D) C
E) Li
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The compound 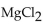 is named
is named
A) dimagnesium chloride.
B) magnesium chloride.
C) magnesium chlorine.
D) magnesium dichloride.
E) magnesium(II) chloride.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The carbon tetrachloride molecule, 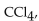 is
is
A) a polar molecule with ionic bonds.
B) a polar molecule with nonpolar bonds.
C) a nonpolar molecule with nonpolar bonds.
D) a nonpolar molecule with polar bonds.
E) a polar molecule with polar bonds.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct formula for the oxide ion?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a covalently bonded molecule, the number of electrons that an atom shares with others is usually equal to the number of electrons
A) in the atom.
B) needed to give it a stable electron configuration.
C) in its nucleus.
D) in all the atoms.
E) in its ion.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of valence electrons found in an atom of a Group A element is equal to
A) its atomic number.
B) its mass number.
C) eight minus the group number.
D) eight.
E) its group number.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ammonia molecule, 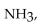 is
is
A) a nonpolar molecule with nonpolar bonds.
B) a polar molecule with ionic bonds.
C) a polar molecule with polar bonds.
D) a nonpolar molecule with polar bonds.
E) a polar molecule with nonpolar bonds.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 83
Related Exams