A) atom
B) element
C) molecule
D) ion
E) compound
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these substances are compounds? I. Neon II. Crude oil III. Water IV. Sodium chloride
A) I
B) I and III
C) II, III, and IV
D) III and IV
E) II and IV
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these statements best explains the law of constant composition?
A) All atoms of a given element have the same weight.
B) Atoms of different elements combine in fixed whole number ratios.
C) The weight of an object is neither created nor destroyed in a chemical reaction.
D) All samples of a given compound have the same proportion of constituent elements.
E) The sum of the masses of the reactants equals the sum of the masses of the products in a normal chemical reaction.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these statements is true?
A) The compositions of both mixtures and pure substances are variable.
B) The compositions of both mixtures and pure substances are fixed.
C) The composition of mixtures is variable, and the composition of pure substances is fixed.
D) The composition of mixtures is fixed, and the composition of pure substances is variable.
E) The compositions of both mixtures and pure substances can be fixed or variable.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The French chemist Antoine Lavoisier found that the weight of objects before burning and the weight of the products after burning were equal. He noticed that the total weight did not change during a process. Which of these best describes the scenario?
A) Lavoisier arrived at a scientific law from observation.
B) Lavoisier arrived at a scientific theory from observation. .
C) Lavoisier arrived at a scientific theory from a scientific law.
D) Lavoisier arrived at a scientific law from a scientific theory.
E) Lavoisier arrived at a scientific conclusion from observation.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The scientific revolution of the 1500s was marked by a move away from _____ and towards _____ as a method for explaining the natural world.
A) law; theory
B) alchemy; research
C) reason; observation
D) scientific theory; experimentation
E) observation; experimentation
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these statements about the scientific method is incorrect ?
A) It is a collection of absolute truths.
B) It uses experiments that are reproducible.
C) It is used for testing claims about the natural world.
D) It requires one to propose a theory and perform experiments to obtain results that confirm or disclaim the theory.
E) It leads to a model of reality from a set of observations.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these statements is true ?
A) Gases are compressible and have a variable shape.
B) Gases are incompressible and have a variable shape.
C) Gases are compressible and have a fixed shape.
D) Gases are incompressible and have a fixed shape.
E) Gases are incompressible and can have a fixed or a variable shape.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
_____ proposed that if a substance could be broken down into simpler substances, it was not an element.
A) Robert Boyle
B) Nicholas Copernicus
C) John Dalton
D) Andreas Vesalius
E) Galileo Galilei
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The smallest unit of a chemical compound is a(n) _____.
A) atom
B) molecule
C) beta particle
D) alpha particle
E) ion
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 12.0 g of carbon react with 32.0 g of oxygen to form 44.0 g of carbon dioxide, which of these statements is false?
A) 18.0 g of carbon is needed to form 66.0 g of carbon dioxide.
B) 48.0 g of oxygen is needed to form 66.0 g of carbon dioxide.
C) 48.0 g of carbon is needed to form 132.0 g of carbon dioxide.
D) 96.0 g of oxygen is needed to form 132.0 g of carbon dioxide.
E) 36.0 g of carbon is needed to form 132.0 g of carbon dioxide.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these are pure substances? I. Steam II. Crude oil III. Salt water IV. Gun powder V. Oxygen VI. Mercury
A) I, II, and III
B) I and III
C) I, II, III, and V
D) I, III, and V
E) I, V, and VI
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the entities that will correctly complete the flow chart. 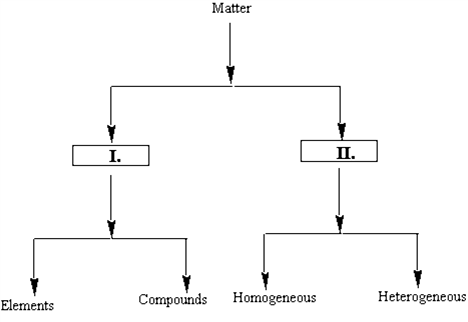

A) I
B) II
C) III
D) IV
E) V
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which two scientists are credited with the endorsement of a sun-centered universe?
A) Dalton and Plato
B) Boyle and Copernicus
C) Copernicus and Galileo
D) Democritus and Vesalius
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true of a chemical compound ?
A) A chemical compound is composed of two or more elements.
B) A chemical compound is a pure substance.
C) A chemical compound has a fixed composition.
D) A chemical compound has a variable composition.
E) The elements present in a compound are different from one another.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following scientists is responsible for establishing the concept of a nuclear atom?
A) Rutherford
B) Proust
C) Dalton
D) Lavoisier
E) Galileo
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following laws best illustrates the following statement? Regardless of the amount of fluorine available, 23 g of sodium always combines with 19 g of fluorine.
A) Law of Constant Composition
B) Law of Conservation of Mass
C) Dalton's Atomic Theory
D) Law of Mass Action
E) Lavoisier's Law
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these sets of masses for nitrogen dioxide is not consistent with the others according to the Law of Constant Composition? 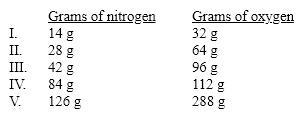
A) I
B) II
C) III
D) IV
E) V
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carbon and oxygen react to form carbon dioxide. What is the mass of carbon dioxide produced when 12.0 g of carbon react with 32.0 g of oxygen?
A) 44 g
B) 38 g
C) 28 g
D) 20 g
E) 2.67 g
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A bowl of chocolate chip ice cream is best described as a _____.
A) pure substance containing only elements
B) homogenous mixture of elements
C) heterogeneous mixture compounds
D) pure substance containing only compounds
E) heterogeneous mixture of elements
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 72
Related Exams