A) From one pH unit below its pKa to its pKa.
B) From its pKa to one pH unit above its pKa.
C) Within one pH unit of its pKa, both above and below.
D) Weak acids do not make good buffers at all.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The substance most likely to form a micelle is
A) acetic acid
B) sodium palmitate
C) methyl alcohol
D) acetone
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules is polar?
A) CCl4
B) CH4
C) CO2
D) NH3
E) None of these molecules is polar.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true about ionic compounds?
A) They are more likely to dissolve in non-polar solvents than covalent compounds.
B) They always dissolve completely in water.
C) They never dissolve in polar solvents.
D) Some of them dissolve completely in water or other polar solvents, while others do not.
F) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
Hydrogen bonds can only form when the hydrogen atom is involved in a polar bond.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The inflection point of the titration curve for a weak monoprotic acid is equal to its pKa
B) False
Correct Answer

verified
True
Correct Answer
verified
Multiple Choice
A buffer solution
A) is used to control the pH of a solution
B) contains at least 100 times more of a weak acid than its conjugate base
C) contains at least 100 times less of a weak acid than its conjugate base
D) always has a pH of 7
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A buffer solution at pH 10 has a ratio of [HA]/[A-] of 10.What is the pKa of the acid?
A) 8
B) 9
C) 10
D) 11
E) 12
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 2B Contains information on the pK's of some common buffers.
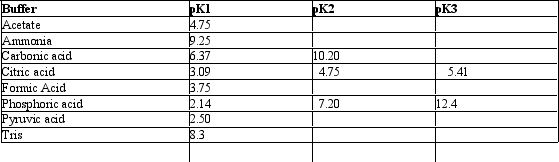 Refer to Exhibit 2B.Which of the following would make the best buffer at pH =10.0?
Refer to Exhibit 2B.Which of the following would make the best buffer at pH =10.0?
A) Acetic acid and sodium acetate
B) Tris and its acid form
C) H2CO3 and NaHCO3
D) Na2HPO4 and NaH2PO4
E) NaHCO3 and Na2CO3
G) None of the above
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
If the pH of 1 liter of a 1.0 M carbonate buffer is 7.0,what is actual number of moles of H2CO3 and HCO3-? (pK = 6.37) 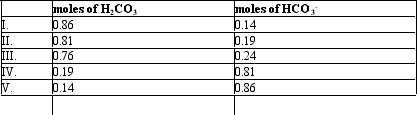
A) I
B) II
C) III
D) IV
E) V
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The tendency for an atom to attract electrons to itself in a chemical bond is called
A) polarity.
B) electronegativity.
C) hydrophilicity
D) electrophilicity.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 2B Contains information on the pK's of some common buffers.
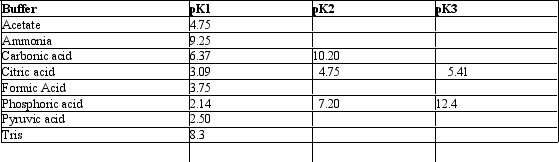 Refer to Exhibit 2B.An ammonium buffer would work well at this pH:
Refer to Exhibit 2B.An ammonium buffer would work well at this pH:
A) 5.6
B) 7.0
C) 9.0
D) 11.0
E) None of these
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a solution has a pH = 6,the [H+] is
A) 6 M
B) 106 M
C) 10-6 M
D) 0.6 M
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a hydrogen bond
A) three atoms lie in a straight line
B) there is stronger bonding than in a covalent bond
C) unpaired electrons play no role
D) none of the above
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The water molecule is polar because:
A) Electrons are not distributed symmetrically in the molecule.
B) The hydrogen atoms are found on one "side" of the molecule.
C) Hydrogen is less electronegative than oxygen.
D) The hydrogen atoms are found on one "side" of the molecule and hydrogen is less electronegative than oxygen.
E) All of these are correct.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true?
A) The pH of a solution where the A- to HA ratio is 1 has a pH = pKa.
B) If the pH does not equal the pKa, the solution is not a buffer.
C) The best buffer for any experiment will always have a pH equal to the pKa.
D) If a buffer has more weak acid than conjugate base, the pH will be higher than the pKa.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum number of hydrogen bonds a single water molecule can form?
A) 1
B) 2
C) 3
D) 4
E) 5
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If atoms with greatly differing electronegativities form a bond,that bond will be
A) polar.
B) nonpolar.
C) amphipathic.
D) acidic.
F) A) and C)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Molecules which contain both hydrophilic and hydrophobic regions are:
A) Amphipathic
B) Detergents
C) Able to form micelles
D) Both amphipathic and detergents.
E) All of these
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ion product constant for water (Kw) is equal to:
A) 1014
B) 107
C) 100
D) 10-7
E) 10-14
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 79
Related Exams