A) 4,5-diethylheptane.
B) 3-propyl-4-ethylhexane.
C) 3-ethyl-4-propylhexane.
D) 3-methyl-4-propylheptane.
E) 2-ethyl-4-propylhexane.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Write the formula for the alcohol and the carboxylic acid from which the following ester may be synthesized. 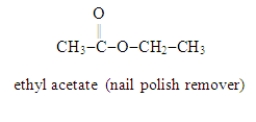
Correct Answer

verified
CH3-COOH and CH3-CH2-OH
Correct Answer
verified
Multiple Choice
The octane rating of gasoline refers to its
A) percentage C8H18 by volume.
B) radiation dose.
C) alcohol level.
D) ability to resist engine knocking.
E) percentage of unsaturated hydrocarbons.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct structure for 2,3,3-trimethylpentane is
A) ![]()
B) ![]()
C) (
![]()
D) ![]()
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Name the following compound: 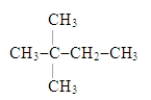
Correct Answer

verified
Correct Answer
verified
True/False
The systematic name for the compound with the following structural formula is 4,5-dimethyl-2-hexene. 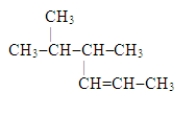
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of these choices is the formula for an aldehyde?
A) CH3CHO
B) CH3OCH3
C) CH3COCH3
D) CH3COOH
E) HC CH
G) A) and E)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
A particular structural isomer of C6H14 is shown below. 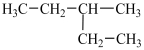 Which of the following structures represents a different structural isomer of C6H14 than the one shown above?
Which of the following structures represents a different structural isomer of C6H14 than the one shown above?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The two molecules represented below are examples of CH3-CH2-O-CH2CH3 CH3CH2CH2CH2-OH
A) geometric isomers.
B) structural isomers.
C) optical isomers.
D) stereoisomers.
E) None of these.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction of ethylene and water yields
A) an aldehyde.
B) an ester.
C) an alcohol.
D) an ether.
E) an organic acid.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The two molecules represented below are examples of 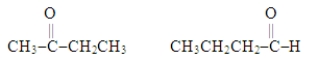
A) isomers.
B) isotopes.
C) alcohols.
D) carboxylic acids.
E) unsaturated hydrocarbons.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of these hydrocarbon chains would have the highest octane rating?
A) ![]()
B) C-C-C-C-C-C
C) ![]()
D) ![]()
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the product of the reaction of one mole of HCl with one mole of 1-butyne?
A) 1-chloro-1-butene
B) 1-chloro-2-butene
C) 2-chloro-1-butene
D) ethyl chloride + acetylene
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these pairs are geometric isomers?
A) CH3CH2-O-CH2CH3 and CH3CH2CH2CH2OH
B) ![]()
C) ![]()
D) ![]()
F) B) and D)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Which choice gives the structures of the reaction products when the ester below is hydrolyzed in acid solution? 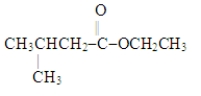
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these is the systematic name for the compound represented below? 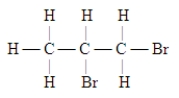
A) 2,3-dibromopentane
B) 1,2-dibromopentane
C) 2,3-dibromopropane
D) 1,2-propane dibromide
E) 1,2-dibromopropane
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name for the compound with the formula CH3CH2CH2CH2OH is
A) propanol.
B) propane.
C) butanol.
D) pentane.
E) pentanol.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these species is an aromatic compound?
A) C2H2
B) C6H12
C) C6H4Br2
D) C5H10
E) C2H4Br2
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxidation of the 2-propanol will produce a/an
A) aldehyde.
B) amine.
C) alkene.
D) ketone.
E) carboxylic acid.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Write the formula for the alcohol and the carboxylic acid from which the following ester may be synthesized. 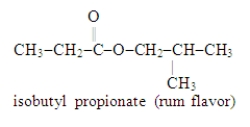
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 57
Related Exams