A) H
B) O
C) N
D) C
E) S
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
There are 8 isomers that have the molecular formula C5H11Br.How many of these are tertiary alkyl bromides?
A) 0
B) 1
C) 2
D) 3
E) 8
G) None of the above
Correct Answer

verified
B
Correct Answer
verified
Essay
Draw the most stable conformer of cis-1-ethyl-3-methylcyclohexane.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following will have the lowest boiling point?
A) CH3Cl
B) CH4
C) CH2Cl2
D) CHCl3
E) CCl4
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Provide the systematic name of the compound shown. 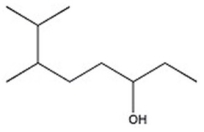
Correct Answer

verified
6,7-dimethyl-3-octanol
Correct Answer
verified
Essay
Draw the Newman projection that represents the least stable conformation of 3,3-dimethylhexane viewed along the C3-C4 bond.
Correct Answer

verified
Correct Answer
verified
Short Answer
Provide an acceptable name for the compound shown below. 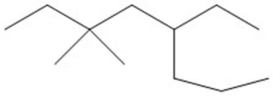
Correct Answer

verified
5-ethyl-3,...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Which of the molecules below has the higher boiling point? Briefly explain your choice. CH3CH2CH2OH or CH3CH2OCH3
Correct Answer

verified
CH3CH2CH2OH has the hig...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
How many axial hydrogens are present in this molecule? 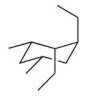
A) 2
B) 3
C) 4
D) 5
E) 6
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Provide an acceptable name for the alkane shown below. 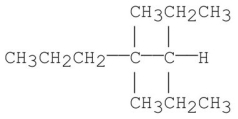
Correct Answer

verified
3-ethyl-4,...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
Provide an acceptable name for the alkane shown below. CH3CH2CH2CH2CH2CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Provide the IUPAC name of the compound. 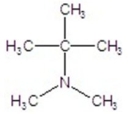
A) N,N,1,1-tetramethylethanamine
B) N,N-dimethyl-2-butanamine
C) N,N,2-trimethyl-1-propanamine
D) N,N,2-trimethylpropanamine
E) N,N,2-trimethyl-2-propanamine
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the staggered conformation for rotation about the C1-C2 bond in the following structure? 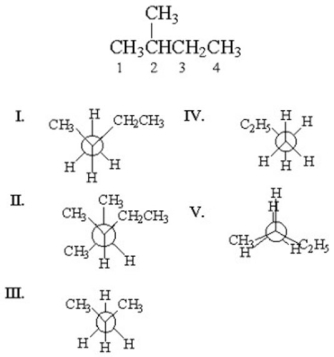
A) I
B) II
C) III
D) IV
E) V
G) All of the above
Correct Answer

verified
A
Correct Answer
verified
Essay
Draw the structure of N-ethyl-5-methyl-3-hexanamine.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the boat conformation of cyclohexane,the "flagpole" hydrogens are located on
A) the same carbon.
B) adjacent carbons.
C) C-1 and C-3.
D) C-1 and C-4.
E) none of the above.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Explain why the molecule shown below has a lower boiling point than CH3CH2CH2CH3. 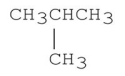
Correct Answer

verified
CH3CH2CH2CH3 has greater van der W...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Arrange the following amines in order of increasing boiling point,lowest bp to highest bp: (CH3)2CHCH2CH2NH2,(CH3)2CHN(CH3)2,and (CH3)2CHCH2NHCH3.
Correct Answer

verified
(CH3)2CHN(CH...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Draw the most stable conformer of cis-1-isopropyl-2-methylcyclohexane.
Correct Answer

verified
Correct Answer
verified
Essay
Define the term conformation.
Correct Answer

verified
Conformations are different ar...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following has the greatest van der Waal's interaction between molecules of the same kind?
A) ![]()
B) CH3CH2CH2CH3
C) ![]()
D) CH3CH2CH2CH2CH3
E) ![]()
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 136
Related Exams