Filters
Question type
A) iron
B) copper
C) granite
D) gold
E) water
F) D) and E)
G) A) and B)
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Question 52
Short Answer
Given the following data: 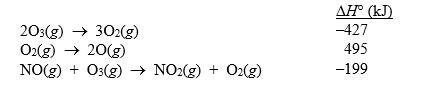 calculate H° for the reaction
NO(g) + O(g) NO2(g)
calculate H° for the reaction
NO(g) + O(g) NO2(g)
Correct Answer

verified
F...View Answer
Show Answer
Correct Answer
verified
F...
View Answer
Question 53
Multiple Choice
Calculate the enthalpy change for the reaction NO(g) + O(g) NO2(g) From the following data: NO(g) + O3(g) NO2(g) + O2(g) H = -198.9 kJ O3(g) 1.5O2(g) H = -142.3 kJ O2(g) 2O(g) H = 495.0 kJ
A) -551.6 kJ
B) -304.1 kJ
C) 190.9 kJ
D) 153.8 kJ
E) 438.4 kJ
F) B) and C)
G) C) and D)
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Question 54
Multiple Choice
A system delivers 1275 J of heat while the surroundings perform 855 J of work on it. Calculate E in J.
A) -2130 J
B) -420 J
C) 420 J
D) 2130 J
E) -1275 J
F) C) and D)
G) All of the above
G) All of the above
Correct Answer

verified
Correct Answer
verified
Question 55
Essay
Although internal energy (E) is more fundamental and conceptually easier than enthalpy (H), in most chemical applications H is more relevant and useful than E. Why?
Correct Answer

verified
Most chemical processes of interest occu...View Answer
Show Answer
Correct Answer
verified
Most chemical processes of interest occu...
View Answer
Showing 81 - 85 of 85
Related Exams