A) " particles"
B) " particles"
C) "neutrons"
D) "positrons"
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the emission that is used to obtain an MRI?
A) alpha
B) beta
C) X-ray
D) none of them
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The new nuclei produced when unstable nuclei undergo radioactive decay is referred to as the _____ .
A) father nuclei
B) daughter nuclei
C) mother nuclei
D) neighbor nuclei
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the responses represent the missing particle in the following reaction? 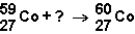
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Ionizing radiation produced fee radicals in living tissue.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Technetium-99m is used in medical diagnosis by injecting a solution and watch for the pattern of emissions.A 0.325 g sample was injected into a person,and the emission rate indicates that there are approximately 0.01016 grams of Tc-99 left.How much time has passed since the injection? Tc-99 has a half-life of 6 hours.
A) 24 hours
B) 30 hours
C) 36 hours
D) Not enough data.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which emissions can be detected by a film badge?
A) X-rays
B) gamma
C) beta
D) all are detected
F) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
One of the symbols for a beta particle is 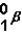 .
.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nuclear emissions are passed through a tunnel that has a positive plate on the top and a negative plate on the bottom.Which of the following emissions is deflected the greatest by the electrical field?
A) " particles
B) " particles"
C) "neutrons"
D) " rays"
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A good radioisotope tracer for medical use should not have the following characteristic.
A) have a long half-life
B) produce penetrating gamma radiation
C) decay to a nontoxic form
D) undergo the same reactions as the nonradioactive element
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the figure below.Which form of emitted radiation would follow the path shown by the arrow labeled 1? 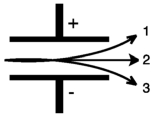
A) " "
B) " -"
C) " "
D) " + "
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
There are some elements within the periodic table that do not occur in nature.One of the man-made elements is _____ .
A) Ta
B) Pt
C) Tc
D) La
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carbon-14 dating is a useful tool for determining the age of the artifacts of ancient civilizations.However,when future archeologists study ruins of our civilization,they obtain confusing results -- with some artifacts dating MUCH older than others.Which of the following would be an explanation for these results?
A) Variations in the Sun's intensity and production of cosmic rays.
B) Ecosystem changes resulting in a reduction in carbon based life.
C) The use of petroleum based synthetic materials.
D) None of the choices.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the nuclear composition of 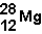 ?
?
A) 12 neutrons and 28 protons
B) 12 neutrons and 16 protons
C) 12 protons and 28 neutrons
D) 12 protons and 16 neutrons
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you are a medical professional and have a chance to be exposed to many sources of radiation,which of the following units to measure biological radiation will most likely be used to express your level of total exposure?
A) roentgen
B) rad
C) gray
D) rem
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What characteristic is important in using radioisotope dating for very old items,such as rocks suspected to be over 100,000,000 years old?
A) The radioisotope cannot be soluble in water.
B) The radioisotope must give off light for easy measurement.
C) The radioisotope in question must decay to another radioisotope.
D) The radioisotope should have a very long half-life.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
A desirable characteristic of a radioisotope used as a tracer is that it produces a radioactive daughter.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sn-108 undergoes electron capture.The product of this reaction would be _____ .
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A patient that works at a nuclear power plant comes to you complaining of nausea and fatigue.You find out that he might have been exposed to a radiation source; what would you recommend be done?
A) Nothing,If he is still alive,there is no real problem.
B) Send the patient home to rest because nausea and fatigue are not signs of a serious exposure.
C) Have additional tests ran to find out the level of exposure.
D) Tell the patient to take two aspirins and call you in the morning,he probably has the flu.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You inject a 160 lb male patient with 1.0 ml of a saline solution containing a radioactive form of sodium.It has an activity of 5.0 * 104 dpm.After allowing sufficient time for the solution to mix,you remove a 1.0 ml sample of blood and measure its radioactivity.You discover it to have an activity of 11 dpm.What is the patient's blood volume? (Hint: this is similar to a M1V1 = M2V2 type problem.)
A) 2.2 liters
B) 3.5 liters
C) 4.6 liters
D) 7.0 liters
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 86
Related Exams