A) increase when temperature increases
B) increase when pH increases
C) are a result of hydrogen bonding
D) are a result of polar covalent bonding
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carbon dioxide (CO₂) is readily soluble in water, according to the equation CO₂ + H₂O ↔ H₂CO₃. Carbonic acid (H₂CO₃) is a weak acid. If CO₂ is bubbled into a beaker containing pure, freshly distilled water, which of the following graphs correctly describes the results?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As the [H3O+] of the solution decreases, the [OH-] ________.
A) increases and thus pH increases
B) increases and thus pH decreases
C) decreases and thus the pH decreases
D) decreases and thus the pH increases
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution contains 0.0000001 (10-7) moles of hydrogen ions [H⁺] per litre. Which of the following best describes this solution?
A) acidic: H⁺ acceptor
B) basic: H⁺ acceptor
C) acidic: H⁺ donor
D) neutral
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a hydrophobic material?
A) paper
B) table salt
C) wax
D) sugar
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following effects can occur because of the high surface tension of water?
A) Rivers cannot freeze solid in winter, despite low temperatures.
B) Spiders can walk across the surface of a small pond.
C) Organisms can resist temperature changes, although they give off heat due to chemical reactions.
D) Sweat can evaporate from the skin, helping to keep people from overheating.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of the compound in the figure are required to make 1 litre of a 0.5 M solution? (Note: The atomic masses, in daltons, are approximately 12 for carbon, 1 for hydrogen and 16 for oxygen.)
A) 30
B) 60
C) 90
D) 120
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the cytoplasm of a cell is at pH 7, and the mitochondrial matrix is at pH 8, then the concentration of H⁺ ions ________.
A) is 10 times higher in the cytoplasm than in the mitochondrial matrix
B) is 10 times higher in the mitochondrial matrix than in the cytoplasm
C) in the cytoplasm is 7/8 the concentration in the mitochondrial matrix
D) in the cytoplasm is 8/7 the concentration in the mitochondrial matrix
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identical heat lamps are arranged to shine on two identical containers, one containing water and one methanol (wood alcohol) , so that each liquid absorbs the same amount of energy minute by minute. The covalent bonds of methanol molecules are nonpolar, so there are no hydrogen bonds among methanol molecules. Which of the following graphs correctly describes what will happen to the temperature of the water and the methanol?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following graphs describes the relationship between [H3O+] and pH?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank, from low to high, the pH of blood, stomach acid, and urine.
A) blood, urine, and stomach acid
B) stomach acid, blood, and urine
C) urine, blood, stomach acid
D) stomach acid, urine, blood
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a solution with a hydroxyl ion (OH-) concentration of 10-10 M?
A) pH 2
B) pH 4
C) pH 10
D) pH 12
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Geraldton, Morowa and Mount Magnet in Western Australia are at about the same latitude, but Mount Magnet has much hotter summers and much colder winters than Geraldton. Why?
A) They are not at the same exact latitude.
B) The ocean near Geraldton moderates the temperature.
C) Fresh water is more likely to freeze than salt water.
D) Mount Magnet is much windier, due to its location in Western Australia.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Melting of ice and thus reduced feeding opportunities for polar bears is occurring because of the ________.
A) increase in phytoplankton population
B) drying up of rivers and streams
C) constant breaking and reforming of hydrogen bonds in water
D) increase in CO₂ and other greenhouse gases in the atmosphere
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
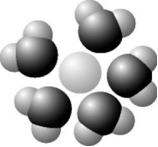 Based on your knowledge of the polarity of water molecules, the solute molecule depicted is most likely ________.
Based on your knowledge of the polarity of water molecules, the solute molecule depicted is most likely ________.
A) positively charged
B) negatively charged
C) without charge
D) nonpolar
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The partial negative charge in a molecule of water occurs because ________.
A) the oxygen atom donates an electron to each of the hydrogen atoms
B) the electrons shared between the oxygen and hydrogen atoms spend more time around the oxygen atom nucleus than around the hydrogen atom nucleus
C) the oxygen atom has two pairs of electrons in its valence shell that are not neutralised by hydrogen atoms
D) one of the hydrogen atoms donates an electron to the oxygen atom
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sulphur is in the same column of the periodic table as oxygen, but has electronegativity similar to carbon. Compared to water molecules, molecules of H₂S will ________.
A) have greater cohesion to other molecules of H₂S
B) have a greater tendency to form hydrogen bonds with each other
C) have a higher capacity to absorb heat for the same change in temperature
D) not form hydrogen bonds with each other
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction at equilibrium: CO₂ + H₂O 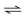 H₂CO₃. What would be the effect of adding additional H₂O?
H₂CO₃. What would be the effect of adding additional H₂O?
A) It would drive the equilibrium dynamics to the right.
B) It would drive the equilibrium dynamics to the left.
C) Nothing would happen because the reactants and products are in equilibrium.
D) Reactions in both the directions will slow down.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How would acidification of seawater affect marine organisms? Acidification of seawater would ________.
A) increase dissolved carbonate concentrations and promote faster growth of corals and shell-building animals
B) decrease dissolved carbonate concentrations and promote faster growth of corals and shell-building animals
C) increase dissolved carbonate concentrations and hinder growth of corals and shell-building animals
D) decrease dissolved carbonate concentrations and hinder growth of corals and shell-building animals
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Water has many exceptional and useful properties. Which is the rarest property among compounds?
A) Water is a solvent.
B) Solid water is less dense than liquid water.
C) Water has a high heat capacity.
D) Water has surface tension.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 55
Related Exams